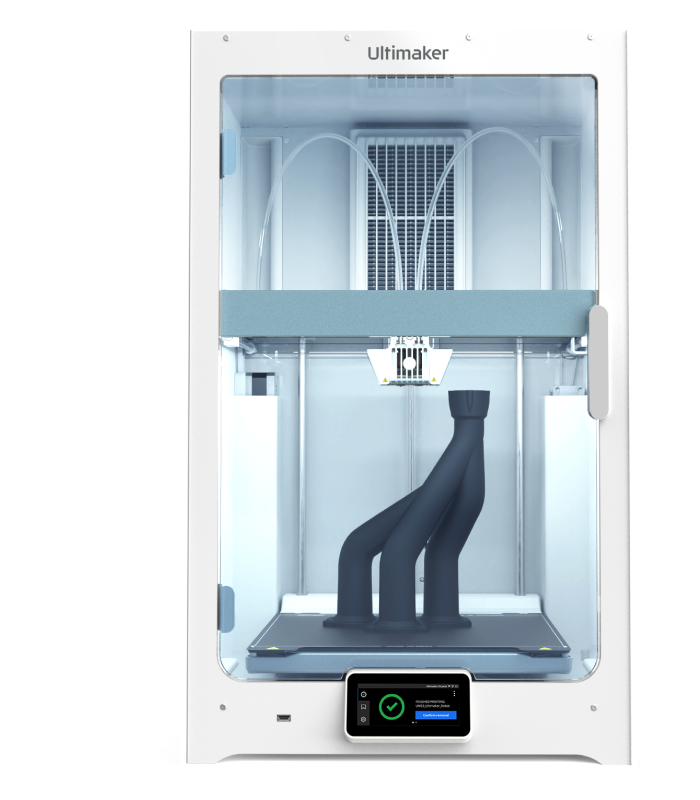
S series 3D printers
Design. Print. Repeat. Unlock high-value applications and reduce costs with our professional 3D printing ecosystem.
Learn more >


Unlimited application potential.

Specialized for engineering applications.

Over 280 materials to choose from.

From polymers to composites to metals.

Optimize production processes

Keeping defense assets operational

A 3D Printer Platform Made for Education

Take your designs to the next level

Explore our support content

Pricing or financing options

Press and general inquiries

Contact our support team directly




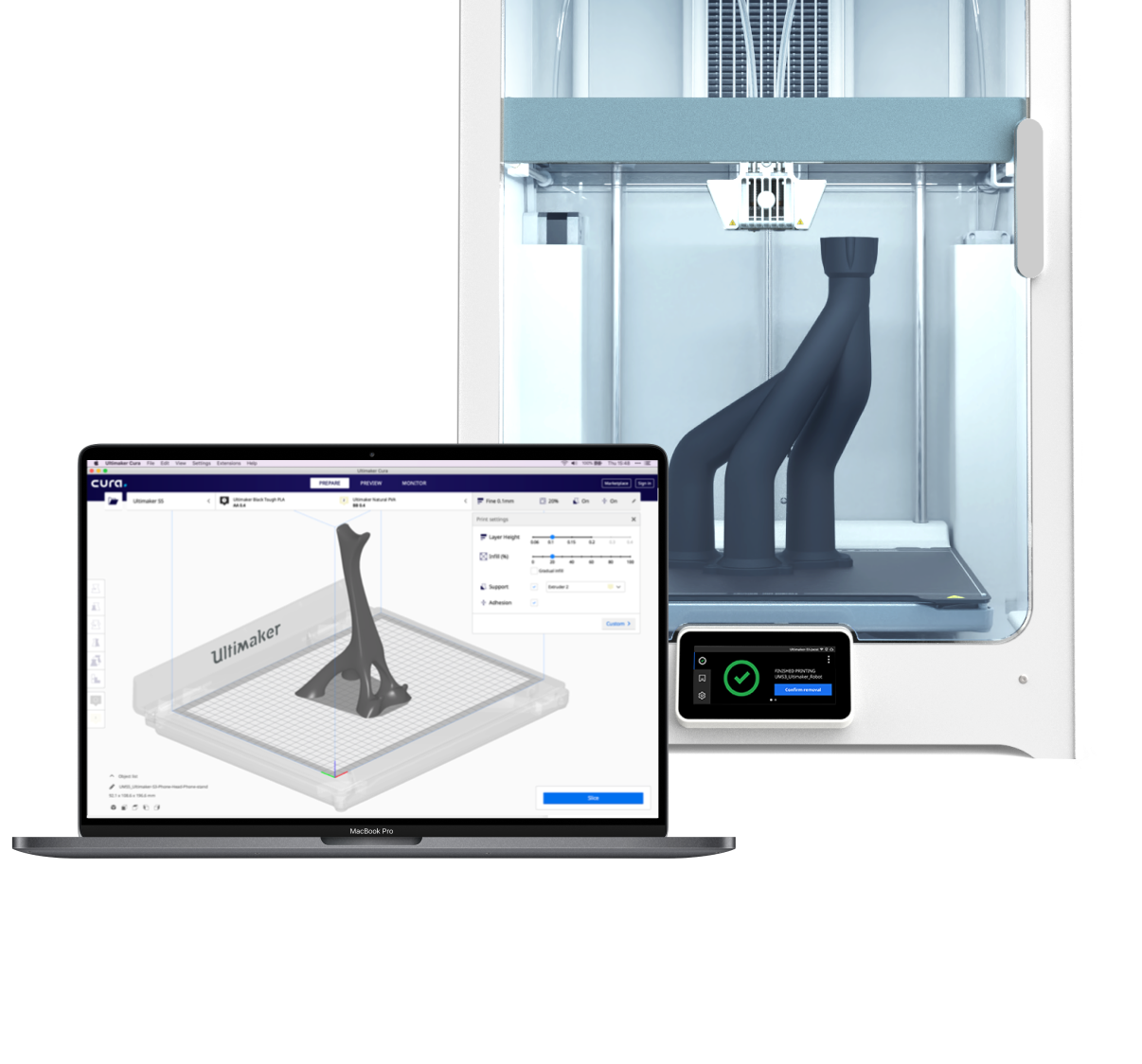
Design. Print. Repeat. Unlock high-value applications and reduce costs with our professional 3D printing ecosystem.

The power of the S series lies in its versatility. Explore new 3D printing applications using the widest choice of materials on the market – making them the perfect for prototyping and manufacturing aids of all sizes. They use 2.85 mm filament and unlock the full power of UltiMaker Cura to simplify your workflow.
Learn more
The Method series is designed for the high-quality production of tools and end-use parts. Their actively heated build chambers, direct drive, and rigid metal frame make it easy to 3D print a specific range of engineering-grade materials with high repeatability and dimensional accuracy using 1.75 mm filament.

The Method series is designed for the high-quality production of tools and end-use parts. Their actively heated build chambers, direct drive, and rigid metal frame make it easy to 3D print a specific range of engineering-grade materials with high repeatability and dimensional accuracy using 1.75 mm filament.
Learn more







The widest choice of high-quality 3D printer filaments on the market. Formulated for S series and Method series 3D printers to achieve results for almost any application you can think of.
S series materials
Trusted by millions of users, UltiMaker Cura is the world’s most popular 3D printing software. Prepare prints with a few clicks, integrate with CAD software for an easier workflow, or dive into custom settings for in-depth control.
Download for free